Abstract
Introduction: CAR T-cells remain in a quiescent or dormant state when unstimulated, showing no proliferative activity. In contrast, upon specific antigen stimulation (i.e., CD19) CAR T-cells divide both in-vitro and in-vivo, initiate immune responses and can kill their target cells in the body. However, one of the major physiological immune changes with increased age is the progressive impairment of T-cell responses. This process termed immunosenescence (which may be similar T-cell exhaustion) is associated with the shortening of telomeres, specific DNA repeated sequences that protect the end of linear chromosomes from degradation and fusion with neighbor chromosomes. We aim to investigate change in T-cell telomere length with CAR-T cell therapy and its potential impact on outcome in patients receiving CART immunotherapy.
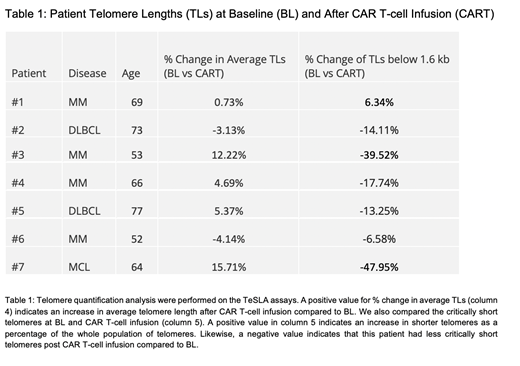
Methods: We enrolled adult patients (age range: 30-80 years old) receiving CART immunotherapy for diffuse large B cell lymphoma (DLBCL), multiple myeloma (MM), mantle cell lymphoma (MCL), or follicular lymphoma (FL). We collected peripheral blood at two time points: i) pre-lymphodepletion therapy and ii) two weeks post CAR-T cell infusion. Peripheral blood mononuclear cells were isolated from blood via density gradient and T-cells isolated from PBMC with magnetic beads (negative selection). Telomere lengths are quantified from T-cells by using a highly sensitive technique called TeSLA (Telomere Shortest Length Assay) that allows absolute quantification of both the average telomere length and the lengths of critically short telomeres, which are believed to play a major role in promoting cell cycle arrest and T-cell exhaustion.
Results: We identified 7 patients receiving CAR T cell therapy for hematological malignancies at University of Texas Southwestern Medical Center. The cohort included 7 patients, 2 patients with DLBCL and 1 patient with MCL receiving CD19 CAR-T Cell therapy and 4 patients with MM receiving BCMA CAR-T cell therapy. Median age of patient was 65 yrs. Median follow up was 273 days post CAR T-cell therapy with all patients being alive at last follow-up. Two patients experienced Grade I Cytokine release syndrome (CRS), two patients with Grade 2 CRS and one patient with Grade 2 ICANS. Our initial analysis shows that patients telomere lengths changes pre and post CAR T-cell infusion. Regarding change in critically short telomere (<1.6kb); 6 out of 7 patients had reduction the shorter telomere from BL to post CAR-T. We are currently evaluating the effect of change in telomere length on outcomes.
Conclusions: CAR T-cell therapy is a game-changer for hematological malignancies; however, disease still relapse. Understanding the mechanics of poor response or relapse after CAR T-cell therapy is critical in advancing the field. Initial results suggest T-cell telomere length are significantly affected during CAR T-cell manufacturing process and post infusion. These results are potentially important as telomere length can be utilized as a biomarker to predict CAR T-cell therapy outcomes.
Anderson: Celgene, BMS, Janssen, GSK, Karyopharm, Oncopeptides, Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Awan: Verastem: Consultancy; Incyte: Consultancy; Cardinal Health: Consultancy; Dava Oncology: Consultancy; BMS: Consultancy; ADCT therapeutics: Consultancy; Beigene: Consultancy; Celgene: Consultancy; Karyopharm: Consultancy; Pharmacyclics: Consultancy; MEI Pharma: Consultancy; Merck: Consultancy; Kite pharma: Consultancy; Gilead sciences: Consultancy; Johnson and Johnson: Consultancy; Abbvie: Consultancy; Janssen: Consultancy; Astrazeneca: Consultancy; Genentech: Consultancy. Madanat: Onc Live: Honoraria; Blue Print Pharmaceutical: Honoraria; Geron Pharmaceutical: Consultancy; Stem line pharmaceutical: Honoraria. Patel: Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; PVI: Honoraria; Agios: Membership on an entity's Board of Directors or advisory committees. Sweetenham: EMA Wellness: Membership on an entity's Board of Directors or advisory committees. Kansagra: Alynylam, Celgene/BMS, Cota Health, GSK, Janssen, Karyopharm, Oncopeptide, Pfizer, Takeda, Sanofi: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal